Research
Our Focus
Our lab works on programs that seek to identify new pharmacological targets for relapse prevention, a notable challenge in the treatment of post-dependent individuals. These projects investigate the neurobiological basis of chronic vulnerability to relapse, with an emphasis on identifying neural substrates that are responsible for the distinctly compulsive nature of drug (i.e., cocaine, alcohol, and opioid) seeking compared with behavior that is motivated by natural rewards. We use animal models of relapse in animals with and without a history of drug dependence. These models include drug-seeking behavior that is induced by cues, stress, and drug priming, all of which can induce intense craving and trigger relapse in abstinent individuals. Using a combination of original molecular techniques (i.e., AAV, DREADDs) and gold-standard animal models, this work has made seminal contributions to the field, showing, for example, that the orexin (hypocretin) system is strongly engaged in drug-seeking behavior. This work also showed that orexin transmission in the paraventricular nucleus of the thalamus (i.e., a region that was not originally thought to be part of “drug addiction” circuitry) is involved in modulating drug-directed behavior.
Topics of Interest
The orexin system.
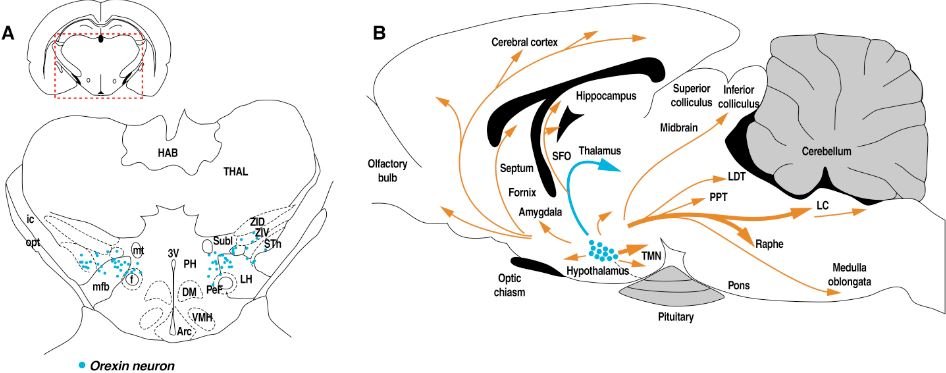
Orexins (also known as hypocretins) participate in the regulation of a range of physiological and behavioral functions (e.g., sleep/wakefulness, arousal, stress, feeding, and energy metabolism). Orexins (i.e., orexin-A and orexin-B) are neuropeptides that derive from a common precursor, prepro-orexin, that is produced exclusively in well-defined subregions of the hypothalamus. Orexin neurons project to key regions that participate in the regulation of drug seeking, including the nucleus accumbens shell, ventral pallidum, ventral tegmental area, central nucleus of the amygdala, bed nucleus of the stria terminalis, medial prefrontal cortex and paraventricular nucleus of the thalamus. Evidence indicates a role for orexin signaling in the neurobehavioral and motivational effects of drugs of abuse and neuroplasticity that is caused by drug exposure may be responsible for maladaptive, compulsive, and addictive behavior.
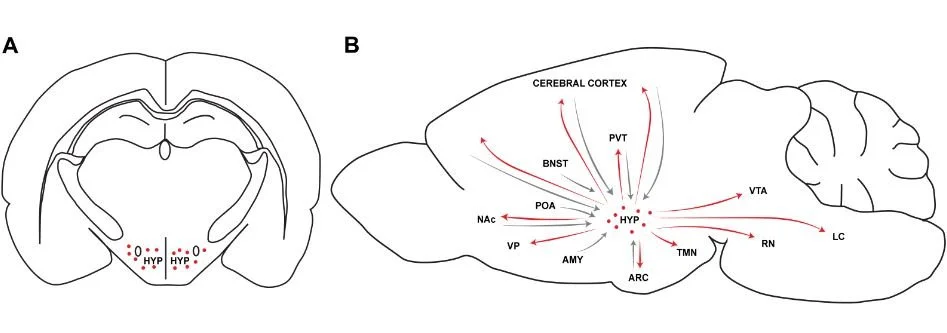
Figure Legend.
A. Coronal rat brain section illustrating the rostrocaudal level of orexin neurons (red dots) in the hypothalamus (HYP)
B. Sagittal section of a rat brain with schematic representation of major hypothalamic orexin efferents (red arrows) and afferents to the HYP (gray arrows) within the neurocircuitry of addiction. HYP, hypothalamus; TMN, tuberomammillary nucleus; PVT, paraventricular nucleus of the thalamus; ARC, arcuate nucleus of the hypothalamus; LC, locus coeruleus; RN, raphe nucleus; VTA, ventral tegmental area; AMY, amygdala; POA, preoptic area; BNST, bed nucleus of the stria terminalis; NAc, nucleus accumbens; VP, ventral pallidum.
Cocaine-motivated behaviors & DREADDs.
The goal is to provide unique information about the specific involvement of the orexin input from the lateral hypothalamus to the infralimbic cortex [LH(Orx) -> IL] in the neurobiology of cocaine-motivated behaviors. The project will investigate the effects of LH(Orx) -> IL activation using a newly developed Gq-Designer Receptor Exclusively Activated by Designer Drugs (Gq-DREADD) and LH(Orx) -> IL inhibition using a newly developed Gi-DREADD on cocaine intake and relapse vs. normal (e.g., food intake and seeking) behavior. The innovative development of viral-based targeting strategies will improve our understanding of the involvement of the orexin system in compulsive-like behaviors that characterize cocaine use disorder.
Restoring the circadian rhythm.
The nonmedical use of prescription opioids, such oxycodone, has emerged as a major concern because of dramatic increases in abuse and overdose. In patients who suffer from prescription opioid use disorder (OUD), insomnia is a major withdrawal symptom. Alleviating sleep disturbances during withdrawal may help reduce relapse vulnerability. We hypothesize that the sleep‐promoting orexin system is compromised by prescription OUD. Our aim is to test the hypothesis that a history of oxycodone dependence dysregulates the orexin system, reflected by the disruption of the physiological circadian rhythm, and that treatment with a dual orexin receptor antagonist will alleviate sleep/wake disturbances and reduce relapse vulnerability.
Mu and kappa opioid receptor and alcohol use disorder.
To understand the importance of opioid peptides and the organization and dynamics of their receptors [μ opioid receptor (MOP) and κ opioid receptor (KOP)] in the central nucleus of the amygdala during alcohol dependence. Specifically, the overall objective of this proposal is to characterize how alcohol perturbs the dynamic lateral organization of signaling domains that harbor MOP and KOP and how OP antagonists stabilize the innate MOP and KOP signaling complexes at the nanoscale level using super-resolution microscopy.
Thalamic orexin transmission and alcohol use disorder.
The goal is to test the hypothesis that a history of alcohol dependence dysregulates orexin and its interaction with the paraventricular nucleus of the thalamus, and that this dysfunction predicts compulsive alcohol seeking (relapse) precipitated by stress. Furthermore, using local gene silencing, a second goal of this proposal is to test the hypothesis that permanent decrease in orexin production via a viral vector could prevent orexin transmission dysregulation in the paraventricular nucleus of the thalamus during dependence and therefore prevent exacerbated response to stress during alcohol abstinence.
Figure modified from Tsujino and Sakurai, Front Behav Neurosci. (2013) 7:28.
Importance of the infralimbic cortex in the dark side of alcohol use disorder.
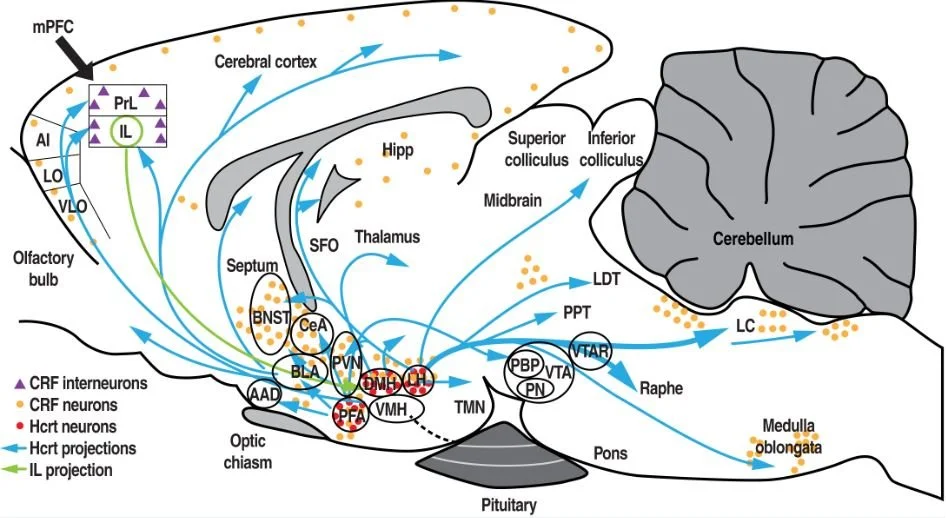
The goal is to provide insights into the specific involvement of orexin/dynorphin/corticotropin-releasing factor in the infralimbic cortex during compulsive alcohol-seeking behavior that is precipitated by stress. From the perspective of future medication development for psychiatric disorders (e.g., drug addiction), this is likely to highlight a previously unrecognized mechanism in the etiology of compulsive alcohol seeking during abstinence, ultimately leading to the identification of novel therapeutic targets for the prevention of alcohol relapse.
Figure from Kim and Martin-Fardon. Alcohol Clin Exp Res. (2020) 44:354-367.